Is Burning Wood Exothermic Or Endothermic
If you ask someone if burning wood is exothermic or endothermic, they may not know the answer. In fact, many people don’t even know what these terms mean. But if you are studying chemistry, it is important to know the difference.
Exothermic reactions are those that release energy in the form of heat, while endothermic reactions absorb heat. So, which is it? Is burning wood exothermic or endothermic?
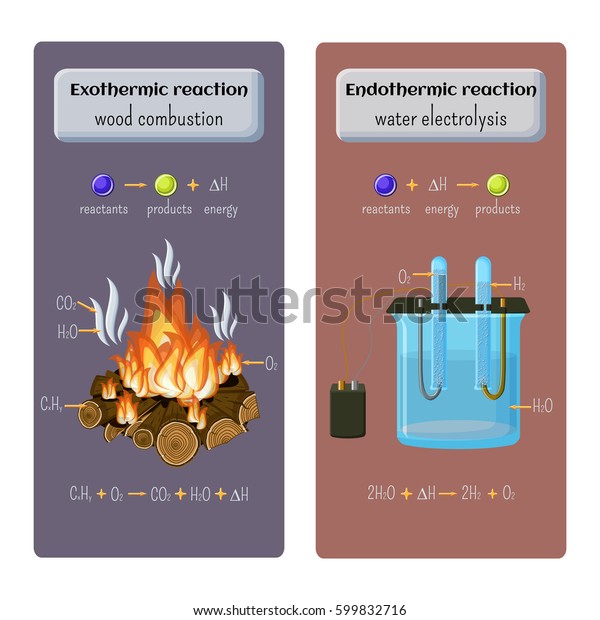
The answer is that burning wood is exothermic. When wood burns, it releases heat and light energy. This happens because the bonds between the atoms in the wood are broken and new bonds are formed.
The breaking of bonds requires energy, and this energy is released as heat and light when new bonds are formed.
When it comes to burning wood, the question of whether the process is exothermic or endothermic can be a bit confusing. In general, burning is a chemical reaction that releases energy in the form of heat and light. However, the specific process of burning wood can be either exothermic or endothermic, depending on the circumstances.
If the wood is burned in an oxygen-rich environment, the process is exothermic and releases a lot of energy. This is how fires typically work – the oxygen in the air fuels the fire and causes it to release heat.
If, however, the wood is burned in an oxygen-poor environment (such as when you smolder a piece of wood rather than setting it ablaze), then the process becomes endothermic and actually absorbs heat from its surroundings.
So, if you’re ever trying to keep a fire going in cold weather, you might want to try smoldering some wood instead!
Is Melting Exothermic Or Endothermic
When a substance melts, the process is called melting or fusion. The temperature at which a solid melts is called the melting point. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure.
When considered without regard to pressure, the melting point for most substances is between 0°C and 100°C (or -173.15°F and 212°F).
The term exothermic means that heat flows from the system into its surroundings during a process. In other words, energy is released by the system as it goes through an exothermic change—such as when ice cream melts on a hot summer day.
Endothermic processes absorb heat from their surroundings; think of how your body cools down when you sweat on a hot day. An endothermic reaction happens when heat flows into the system from its surroundings during a chemical reaction. In general, any time two substances combine to form one new substance, there’s an endothermic reaction taking place.
Credit: www.shutterstock.com
Why is Burning Wood Exothermic?
When you burn wood, the combustion reaction is exothermic. This means that it releases heat. The heat is released because the bonds between the atoms in the wood are broken and new bonds are formed.
When bonds are broken, energy is released. When new bonds are formed, energy is absorbed. The overall effect is that burning wood releases more energy than it absorbs, so it gives off heat.
The reason that burning wood is exothermic has to do with the types of bonds that are broken and formed during combustion. Wood is made up of cellulose molecules, which have a lot of carbon-oxygen double bonds. These double bonds are very strong, so breaking them requires a lot of energy.
But once they’re broken, the oxygen atoms can bond to other oxygen atoms (forming O2 molecules) and the carbon atoms can bond to other carbon atoms (forming CO2 molecules). These new bonds aren’t as strong as the double bonds in cellulose, so forming them releases energy. That’s why burning wood is exothermic: more energy is released when the strong double Bonds in cellulose are broken than when the new weaker Bonds are formed.
What Type of Reaction is Burning Wood?
When you burn wood, the heat from the fire causes a chemical reaction between the oxygen in the air and the cellulose in the wood. The cellulose breaks down into carbon dioxide and water vapor, and these gases are released into the air. The heat from the fire also causes the volatile chemicals in the wood to break down into simpler molecules, which are released as gases.
These gases include carbon monoxide, hydrogen gas, methane, and other hydrocarbons.
Is Wood Burning Endergonic?
When it comes to wood burning, the answer isn’t as simple as whether or not the process is endergonic. In fact, there are a few different things to consider when trying to determine if wood burning is endergonic.
First, it’s important to understand what endergonic reactions are.
These are chemical reactions that require energy input in order to proceed. In other words, they’re not spontaneous and can’t happen without some sort of push.
Second, we need to consider the specific reaction that’s taking place when wood is burned.
When wood burns, it undergoes combustion – meaning that oxygen from the air reacts with the hydrocarbons in the wood to create heat, light, water vapor, and carbon dioxide.
So does this mean that burning wood is an endergonic reaction?
In general terms, yes – but there’s a little more to it than that.
See, while the overall reaction may be endergonic, individual steps within that reaction can be exothermic – meaning they release energy rather than absorb it.
It’s these exothermic steps that make combustion possible without an external source of energy (like a match).
So while burning wood may be an endergonic overall reaction, it doesn’t require energy input in order to occur because certain steps within that reaction actually release energy.
What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchool
Conclusion
When it comes to burning wood, the debate between whether the process is exothermic or endothermic rages on. However, the answer may surprise you.
While both processes release energy, wood burning is actually exothermic.
This is because the process of combustion creates more heat than it consumes. The heat produced by combustion is what makes fire so hot.
So, when you’re sitting around a campfire roasting marshmallows, know that the wood burning process is helping to keep you warm!






